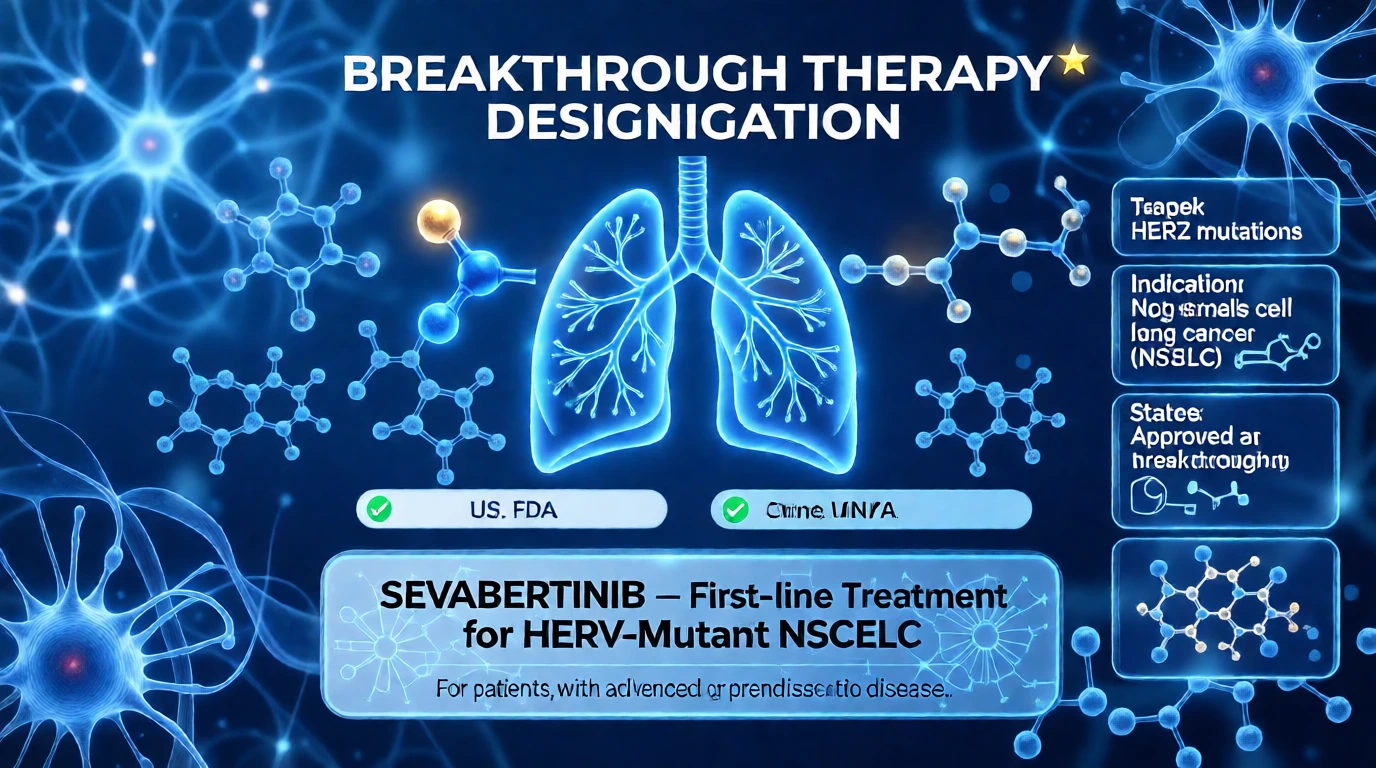
U.S. and China Grant Breakthrough Therapy Designation to Bayer’s Sevabertinib as First-Line Therapy for HER2-Mutant Lung Cancer
The U.S. Food and Drug Administration (FDA) and China’s Center for Drug Evaluation (CDE) have granted Breakthrough Therapy Designation to sevabertinib for first-line treatment of patients with HER2-mutant non-small cell lung cancer (NSCLC), recognizing its potential to offer substantial improvement over existing therapies in a setting of high unmet medical need. This latest regulatory recognition follows the FDA’s recent accelerated approval of sevabertinib for previously treated patients with advanced HER2-mutant NSCLC
Bayer has announced that both the U.S. Food and Drug Administration (FDA) and China’s Center for Drug Evaluation (CDE) have granted Breakthrough Therapy Designation to sevabertinib as a first-line treatment for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring activating mutations in the human epidermal growth factor receptor 2 (HER2) gene.Sevabertinib is an oral, reversible, small-molecule tyrosine kinase inhibitor (TKI) specifically designed to target HER2-activating mutations.
“Receiving Breakthrough Therapy Designation from both the FDA and China’s CDE for sevabertinib as a potential first-line option in advanced HER2-mutant NSCLC underscores its promise in addressing a disease with historically limited therapeutic choices and a poor prognosis,” said Dr. Christian Rommel, Head of Research and Development for Bayer’s Pharmaceuticals Division. “This latest regulatory recognition follows closely on the heels of the FDA’s accelerated approval of sevabertinib in November 2025 for previously treated patients with advanced HER2-mutant NSCLC. Together, these milestones reflect Bayer’s dedication to advancing precision medicine that meets urgent unmet needs and aims to significantly improve survival and quality of life for patients.”
The Breakthrough Therapy Designations are based on early clinical results from cohort F of the ongoing Phase I/II SOHO-01 study (NCT05099172), which is evaluating sevabertinib’s safety and efficacy in treatment-naïve patients with locally advanced or metastatic NSCLC driven by HER2-activating mutations. Breakthrough Therapy Designation is a regulatory pathway intended to accelerate the development and review of drugs that show substantial improvement over existing therapies—or provide the first effective treatment—for serious or life-threatening conditions.
In November 2025, sevabertinib, marketed under the brand name Hyrnuo™, received accelerated FDA approval for patients with previously treated advanced HER2-mutant NSCLC. That decision was supported by data from cohorts D and E of the SOHO-01 trial, which assessed objective response rate (ORR) and duration of response (DOR) in patients who had either not received prior HER2-targeted therapy or had previously been treated with HER2-directed antibody-drug conjugates.
About sevabertinib
Sevabertinib is a new, oral, reversible, small molecule, tyrosine kinase inhibitor (TKI) that potently inhibits mutant human HER2, including HER2 exon 20 insertions and HER2 point mutations, as well as epidermal growth factor receptors (EGFR), with high selectivity for mutant vs wild-type EGFR. Sevabertinib works by blocking certain enzymes called tyrosine kinases, which are involved in the growth of cancer cells. Reversible TKIs can temporarily block their targets, allowing for more precise control over treatment and potentially reducing long-term side effects compared to irreversible TKIs.
Sevabertinib is derived from Bayer’s strategic research alliance with the Broad Institute of MIT and Harvard in Cambridge, MA, USA. As part of its clinical development program, alongside the SOHO-01 trial, sevabertinib is also being studied in patients in the Phase III SOHO-02 trial (NCT 06452277) evaluating sevabertinib in treatment naïve patients with HER2-mutant NSCLC and in the panSOHO study (NCT06760819) in patients with metastatic or unresectable solid tumors harboring HER2-activating mutations, excluding advanced NSCLC.
About Non-Small Cell Lung Cancer (NSCLC)
Lung cancer is the leading cause of cancer-related deaths worldwide. NSCLC is the most common type of lung cancer, accounting for more than 85% of cases. Activating HER2 mutations are found in 2% to 4% of patients with advanced NSCLC. 80% of people diagnosed with NSCLC have already progressed to advanced stages, which makes it more difficult to treat. Patients with HER2-mutant NSCLC currently face limited treatment options, highlighting an urgent need for more effective therapies.
About Oncology at Bayer
Bayer is committed to delivering science for a better life by advancing a portfolio of innovative treatments. The company has the passion and determination to develop new medicines that help improve and extend the lives of people living with cancer. The oncology franchise at Bayer includes several marketed products across diverse indications and multiple compounds in different stages of clinical development. We have a wealth of expertise in areas including: Tumor Intrinsic Pathways, Targeted Radionuclide Therapies, and Next-Generation Immuno-Oncology. We are advancing prostate cancer treatment from early to metastatic stage, with the goal of extending survival while limiting side effects. Part of Bayer’s focus on innovative precision oncology treatments, includes an approved TRK inhibitor exclusively designed to treat tumors that have an NTRK gene fusion, the oncogenic driver of tumor growth and spread.
About Bayer
Bayer is a global enterprise with core competencies in the life science fields of health care and nutrition. In line with its mission, “Health for all, Hunger for none,” the company’s products and services are designed to help people and the planet thrive by supporting efforts to master the major challenges presented by a growing and aging global population. Bayer is committed to driving sustainable development and generating a positive impact with its businesses. At the same time, the Group aims to increase its earning power and create value through innovation and growth. The Bayer brand stands for trust, reliability and quality throughout the world. In fiscal 2024, the Group employed around 93,000 people and had sales of 46.6 billion euros. R&D expenses amounted to 6.2 billion euros.
Source link: https://www.bayer.com/